
Image: Methylene blue inhibits nucleation and elongation of SOD1 amyloid fibrils
In the last few substacks I have been talking about prion like proteins, Amyloid like polymers, how they are used in Nanotechnology and the relationship to the C19 bioweapon. This is to explain the recent research findings by Dr. Diana Wojtkowiak that she did on the deceased C19 vaccinated blood clots. I compared this to the research of Clifford Carnicom and myself showing polyamide protein polymers.I have also compared to my findings in live blood analysis and one can see the apparent similarities:
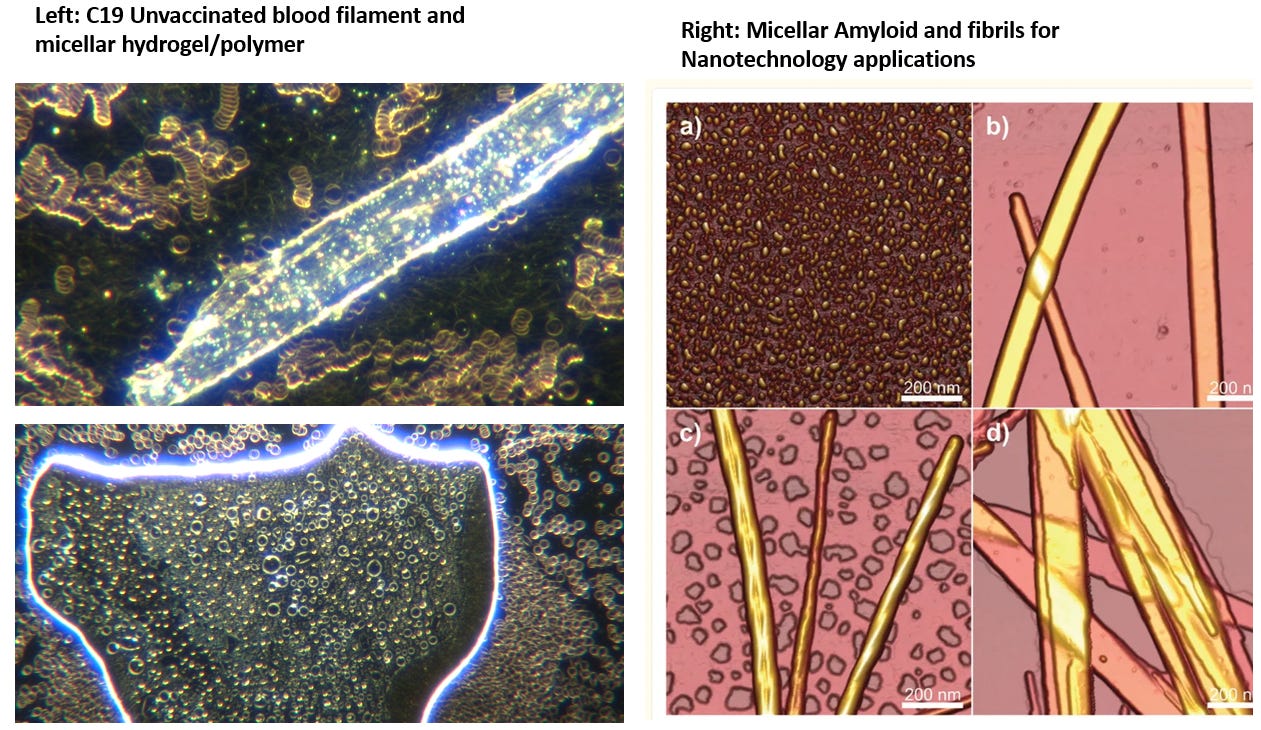
The Russian Study was published showing self assembly amyloid like nanostructures from Sars Cov S1. I wanted let you know in this article about further research on Methylene Blue(MB). It dissolves all of these named culprits. I have posted the research articles below. MB has long been used for reversal of neurocognitive symptoms and I have for years had great clinical success with this molecule in my patients. In the studies below you can see that it prevents amyloid from forming but in animal studies also has been shown to reverse the amyloid that is already there. It works the same for any prion disease, because they are both caused by the misfolding of proteins.
Methylene Blue has been shown in extensive research on prevention of cognitive decline, dissolution and prevention of prions and amyloid and its many other health benefits including its use in cancer treatment – which in light of the rising Turbo cancers is a beneficial and noteworthy consideration.
These are the recent posts discussing the findings of amyloid prions associated with the C19 bioweapons and associated rubbery clots.
Here are the articles discussing Methylene Blue as an inhibitor of amyloid and prion production:
In this study all aspects of prion protein production are inhibited by MB.
Abstract
Neurodegenerative protein misfolding diseases, including prionopathies, share the common feature of accumulating specific misfolded proteins, with a molecular mechanism closely related. Misfolded prion protein (PrP) generates soluble oligomers that, in turn, aggregate into amyloid fibers. Preventing the formation of these entities, crucially associated with the neurotoxic and/or infectious properties of the resulting abnormal PrP, represents an attractive therapeutic strategy to ameliorate prionopathies. We focused our attention into methylene blue (MB), a well-characterized drug, which is under study against Alzheimer’s disease and other neurodegenerative disorders. Here, we have undertaken an in vitro study on the effects of MB on oligomerization and fibrillization of human, ovine and murine PrP. We demonstrated that MB affects the kinetics of PrP oligomerization and reduces the amount of oligomer of about 30%, in a pH-dependent manner, by using SLS and DSC methodologies. Moreover, TEM images showed that MB completely suppresses fiber formation at a PrP:MB molar ratio of 1:2. Finally, NMR revealed a direct interaction between PrP and MB, which was mapped on a surface cleft including a fibrillogenic region of the protein. Our results allowed to surmise a mechanism of action in which the MB binding to PrP surface markedly interferes with the pathway towards oligomers and fibres. Therefore MB could be considered as a general anti-aggregation compound, acting against proteinopathies.
This study also discussed MB as a therapeutic agent and neuroprotection. This is applicable to anyone who has brainfog, since that symptoms is a sign of accelerated cognitive impairment induced by the toxicity of the C19 bioweapon and its shedding. Remember that I found in the live blood analysis that Covid is acute poisoning of the blood with these self assembly structures.
Molecular Mechanisms of the Neuroprotective Effect of Methylene Blue
…but there are premises for its repurposing as a neuroprotective agent based on the efficacy of this compound demonstrated in the models of Alzheimer’s, Parkinson’s, and Huntington’s diseases, traumatic brain injury, amyotrophic lateral sclerosis, depressive disorders, etc.
In this below article the MB promotes fibrillization which has been shown to be better dissolved and these are less toxic to the body. However, since this study others have shown that MB can disolve the structures. In the article above they evolved the research and showed it helps in all aspects of inhibiting the amyloid polymer formation.
Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization
Methylene blue inhibited oligomerization when used at substoichiometric concentrations relative to that of the Abeta monomer. Inhibition of Abeta oligomerization was achieved concomitant with promotion of fibrillization, suggesting that oligomer and fibril formation are distinct and competing pathways. Methylene blue-mediated promotion of fiber formation occurred via a dose-dependent decrease in the lag time and an increase in the fibrillization rate, consistent with promotion of both filament nucleation and elongation. Addition of methylene blue to preformed oligomers resulted in oligomer loss and promotion of fibrillization. The data show that Abeta oligomer formation is inhibited by promoting fibril formation, which suggests that the relative pathological significance of oligomers and fibrils may be tested in vivo using methylene blue. If Abeta oligomers represent the primary pathogenic species, then inhibition of this highly toxic species via promotion of formation of less toxic aggregates may be therapeutically useful.
This next article also discusses different mechanisms on how MB inhibits the amyloid fibrils or filaments to form.
Exploring the effects of methylene blue on amyloid fibrillogenesis of lysozyme
The 129-residue lysozyme has been shown to form amyloid fibrils in vitro. While methylene blue (MB), a compound in the phenothiazinium family, has been shown to dissemble tau fibril formation, its anti-fibrillogenic effect has not been thoroughly characterized in other proteins/peptides. This study examines the effects of MB on the in vitro fibrillogenesis of lysozyme at pH 2.0 and 55 °C. Our results demonstrated that, upon 7-day incubation, the plateau ThT fluorescence of the sample was found to be ~8.69% or ~2.98% of the control when the molar ratio of lysozyme to MB was at 1:1.11 or 1:3.33, respectively, indicating that the inhibitory potency of MB against lysozyme fibrillogenesis is positively correlated with its concentration. We also found that MB is able to destabilize the preformed lysozyme fibrils. Moreover, molecular docking and molecular dynamics simulations results revealed that MB’s mechanism of fibril formation inhibition may be triggered by binding with lysozyme’s aggregation-prone region. Results reported here provide solid support for MB’s effect on amyloid fibrillogenesis.
MB was also noted as a successful therapeutic agent in this review paper:
Here is further research that shows the elongation of the filaments is inhibited as well as other aggregate structures:
Methylene blue inhibits nucleation and elongation of SOD1 amyloid fibrils
Protein aggregation into highly-structured amyloid fibrils is linked to several neurodegenerative diseases. Such fibril formation by superoxide dismutase I (SOD1) is considered to be related to amyotrophic lateral sclerosis, a late-onset and fatal disorder. Despite much effort and the discovery of numerous anti-amyloid compounds, no effective cure or treatment is currently available. Methylene blue (MB), a phenothiazine dye, has been shown to modulate the aggregation of multiple amyloidogenic proteins. In this work we show its ability to inhibit both the spontaneous amyloid aggregation of SOD1 as well as elongation of preformed fibrils.
In this article the mechanism of how MB inhibits amyloid formation in Alzeimer’s disease is discussed.
The phenothiazine methylene blue (MB) is attracting increasing attention because it seems to have beneficial effects in the pathogenesis of Alzheimer’s disease (AD). Among other factors, the presence of neuritic plaques of amyloid-β peptide (Aβ) aggregates, neurofibrilar tangles of tau and perturbation of cytosolic Ca2+ are important players of the disease. It has been proposed that MB decreases the formation of neuritic plaques due to Aβ aggregation. However, the molecular mechanism underlying this effect is far from clear. In this work, we show that MB stimulates the Ca2+-ATPase activity of the plasma membrane Ca2+-ATPase (PMCA) in human tissues from AD-affected brain and age-matched controls and also from pig brain and cell cultures. In addition, MB prevents and even blocks the inhibitory effect of Aβ on PMCA activity. Functional analysis with mutants and fluorescence experiments strongly suggest that MB binds to PMCA, at the C-terminal tail, in a site located close to the last transmembrane helix and also that MB binds to the peptide. Besides, Aβ increases PMCA affinity for MB.
This is an animal study that shows MB actually reverses the damage that was already done by amyloid deposits in the brain and it improves brain function.
Amyloid precursor protein (APP) proteolysis is required for production of amyloid-β (Aβ) peptides that comprise β-amyloid plaques in the brains of patients with Alzheimer disease (AD). Here, we tested whether the experimental agent methylene blue (MB), used for treatment of methemoglobinemia, might improve AD-like pathology and behavioral deficits. We orally administered MB to the aged transgenic PSAPP mouse model of cerebral amyloidosis and evaluated cognitive function and cerebral amyloid pathology. Beginning at 15 months of age, animals were gavaged with MB (3 mg/kg) or vehicle once daily for 3 months. MB treatment significantly prevented transgene-associated behavioral impairment, including hyperactivity, decreased object recognition, and defective spatial working and reference memory, but it did not alter nontransgenic mouse behavior. Moreover, brain parenchymal and cerebral vascular β-amyloid deposits as well as levels of various Aβ species, including oligomers, were mitigated in MB-treated PSAPP mice. These effects occurred with inhibition of amyloidogenic APP proteolysis. Specifically, β-carboxyl-terminal APP fragment and β-site APP cleaving enzyme 1 protein expression and activity were attenuated. Additionally, treatment of Chinese hamster ovary cells overexpressing human wild-type APP with MB significantly decreased Aβ production and amyloidogenic APP proteolysis. These results underscore the potential for oral MB treatment against AD-related cerebral amyloidosis by modulating the amyloidogenic pathway.
I have written extensively about photodynamic therapy with MB in my book Light Medicine – A New Paradigm – The Science of Light, Spirit and Longevity. The below study shows that light activated MB inhibits the self assembly amyloid polymers and dissolves their structures.
Abnormal aggregation of β-amyloid (Aβ) peptides is a major hallmark of Alzheimer’s disease (AD). In spite of numerous attempts to prevent the β-amyloidosis, no effective drugs for treating AD have been developed to date. Among many candidate chemicals, methylene blue (MB) has proved its therapeutic potential for AD in a number of in vitro and in vivo studies; but the result of recent clinical trials performed with MB and its derivative was negative. Here, with the aid of multiple photochemical analyses, we first report that photoexcited MB molecules can block Aβ42 aggregation in vitro. Furthermore, our in vivo study using Drosophila AD model demonstrates that photoexcited MB is highly effective in suppressing synaptic toxicity, resulting in a reduced damage to the neuromuscular junction (NMJ), an enhanced locomotion, and decreased vacuole in the brain. The hindrance effect is attributed to Aβ42 oxidation by singlet oxygen (1O2) generated from photoexcited MB. Finally, we show that photoexcited MB possess a capability to disaggregate the pre-existing Aβ42 aggregates and reduce Aβ-induced cytotoxicity. Our work suggests that light illumination can provide an opportunity to boost the efficacies of MB toward photodynamic therapy of AD in future.
Here are other articles regarding my observations and research of Methylene Blue as a powerful and helpful molecules in these trying times. I had also previously discussed the effects on polymer hydrogels in the below articles:
Methylene Blue Prevents Rubbery Clot Formation, Essential Oils Help Too – Experiment Documentation
Of note, in the Torsion Spectroscopy of the clots, Copper, Zinc and Selenium were found. In this study, EDTA blocks the metal interaction with the Amyloid, hence promotes dissolution. I still highly recommend EDTA as I have found it to be clinically very helpful, to remove the metal contaminants that are contributing to the construction of the nanotechnology biosensors.
The accumulation of the β-amyloid (Aβ) aggregates induced by Cu2+/Zn2+ in conjunction with toxicity is closely related to Alzheimer’s disease (AD). Herein, we intended to improve the efficiency and selectivity of traditional chelator ethylenediaminetetraacetic acid (EDTA) combined with a fluorescent group 4-aminosalicylic acid (4-ASA)to acquire a novel potential chelator 4,4′-((2,2′-(ethane-1,2-diylbis((carboxymethyl)azanediyl))bis(acetyl))bis(azanediyl))bis(2-hydroxybenzoic acid) (EDTA-ASA) capable of disaggregating Aβ-Cu(II)/ Zn(II) aggregates. EDTA-ASA combines 4-ASA as fluorophore and multidentate amino nitrogen, hydroxyl and carboxyl groups to chelate Cu2+ from Aβ-Cu (II) aggregates. The specific selectivity of EDTA-ASA towards Cu2+ in Tris-HCl buffer solution was investigated by fluorescence measurements. It exhibits high recognition towards Cu2+ with no significant interference of other competitive metal ions, which overcomes the deficiencies of EDTA. Importantly, the binding sites and binding mode for Cu2+ were clarified through DFT calculations. The thioflavin-T (ThT) fluorescence analyses and transmission electron microscopy (TEM) results have revealed EDTA-ASA exhibited an enhanced disaggregation capability on Aβ-Cu (II)/Zn (II) aggregates in comparison to EDTA. The Cu2+ chelating affinity was sufficient for EDTA-ASA to sequester Cu2+ from Aβ-Cu (II) aggregates.
Summary:
In this article, I review multiple studies that discuss Methylene Blue as a dissolver of amyloid and prion polymers which are not only created by the body “naturally” but can be externally introduced for nano technological purposes. Prions are highly infectious. Both Amyloid and Prion like peptides have been used to build hydrogel, and nano technological devices and biosensors. These are the building blocks for the Brain Computer Interface and WBAN bidirectional telemetry surveillance.
Methylene Blue should be considered as a preventative and therapeutic option in combination with high dose Vitamin C, EDTA and other supportive treatments for the inhibition and dissolution of the rubbery clot formation, C19 bioweapon induced prion disease and amyloidosis, as well as a potent dissolver of the self assembly nanotechnology assault on humanity.


