Morphology and composition of incrustants in different conditions. (a) Scanning electron microscopic (SEM) images of bare-polystyrene (PS, 1 μm, 1 mg L–1) and incrustant coprecipitates formed in tap water at different temperatures (180 mg L–1 of CaCO3, 40 mL, 25–100 oC); (b) SEM images of bare-PS (1 μm, 1 mg L–1) and incrustant coprecipitates in different water hardness upon boiling (60–300 mg L–1 of CaCO3, 100 oC); (c) SEM images of bare-PS and incrustant coprecipitates in different PS concentrations (1 μm, 0–5 mg L–1) upon boiling of tap water (180 mg L–1 of CaCO3, 100 oC); and (d) SEM images and (e) X-ray diffraction patterns of bare-, carboxyl-, and amino-PS and incrustant coprecipitates upon boiling of tap water (1 and 0.1 μm, 1 mg L–1, 180 mg L–1 of CaCO3, 100 oC).
____________________________________________________________________________
This is a hopeful article explaining the methodology to decontaminate drinking water. This is very important because we do know that all bottled water is contaminated. You can read that study here:
The abstract states:
Tap water nano/microplastics (NMPs) escaping from centralized water treatment systems are of increasing global concern, because they pose potential health risk to humans via water consumption. Drinking boiled water, an ancient tradition in some Asian countries, is supposedly beneficial for human health, as boiling can remove some chemicals and most biological substances. However, it remains unclear whether boiling is effective in removing NMPs in tap water. Herein we present evidence that polystyrene, polyethylene, and polypropylene NMPs can coprecipitate with calcium carbonate (CaCO3) incrustants in tap water upon boiling. Boiling hard water (>120 mg L–1 of CaCO3) can remove at least 80% of polystyrene, polyethylene, and polypropylene NMPs size between 0.1 and 150 μm. Elevated temperatures promote CaCO3 nucleation on NMPs, resulting in the encapsulation and aggregation of NMPs within CaCO3 incrustants. This simple boiling-water strategy can “decontaminate” NMPs from household tap water and has the potential for harmlessly alleviating human intake of NMPs through water consumption.
Here is the ACS article:
Drinking Boiled Tap Water Reduces Human Intake of Nanoplastics and Microplastics
Here is the sciencedaily write up:
Want fewer microplastics in your tap water? Try boiling it first
Contamination of water supplies with nano- and microplastics (NMPs), which can be as small as one thousandth of a millimeter in diameter or as large as 5 millimeters, has become increasingly common. The effects of these particles on human health are still under investigation, though current studies suggest that ingesting them could affect the gut microbiome. Some advanced drinking water filtration systems capture NMPs, but simple, inexpensive methods are needed to substantially help reduce human plastic consumption. So, Zhanjun Li, Eddy Zeng and colleagues wanted to see whether boiling could be an effective method to help remove NMPs from both hard and soft tap water.
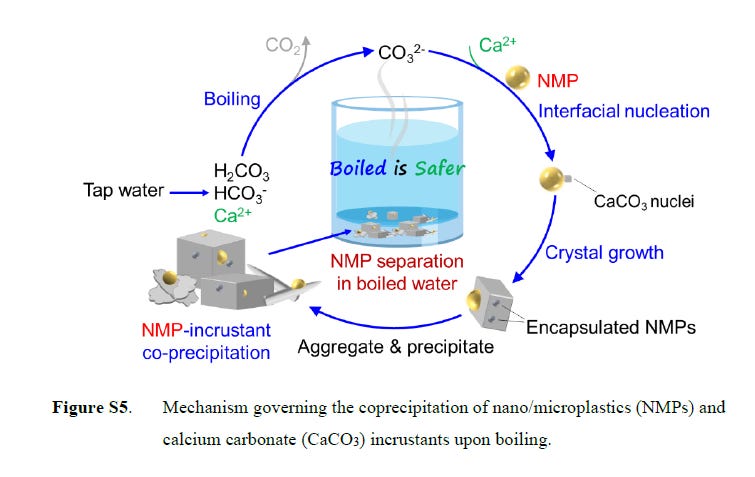
The researchers collected samples of hard tap water from Guangzhou, China, and spiked them with different amounts of NMPs. Samples were boiled for five minutes and allowed to cool. Then, the team measured the free-floating plastic content. Boiling hard water, which is rich in minerals, will naturally form a chalky substance known as limescale, or calcium carbonate (CaCO3). Results from these experiments indicated that as the water temperature increased, CaCO3 formed incrustants, or crystalline structures, which encapsulated the plastic particles. Zeng says that over time, these incrustants would build up like typical limescale, at which point they could be scrubbed away to remove the NMPs. He suggests any remaining incrustants floating in the water could be removed by pouring it through a simple filter such as a coffee filter.
In the tests, the encapsulation effect was more pronounced in harder water — in a sample containing 300 milligrams of CaCO3 per liter of water, up to 90% of free-floating MNPs were removed after boiling. However, even in soft water samples (less than 60 milligrams CaCO3 per liter), boiling still removed around 25% of NMPs. The researchers say that this work could provide a simple, yet effective, method to reduce NMP consumption.
From the paper supplemental information
Results. Boiling hard water can remove most PS, PE, and PP MPs, and PS, PE, and PP MPs precipitation efficiencies were 95 ± 4%, 81 ± 3%, and 90 ± 3%, respectively, at 100 oC. Increasing temperature accelerated the formation of incrustants on spherical, fragmented, and fibrous MP surfaces. MPs continued to be encapsulated by newly formed incrustants (Figure S2) and finally precipitated under gravity, confirming that spherical PS, fragmented PE, and fibrous PP MPs are able to coprecipitate with incrustants in tap water upon boiling. In concluding, the results with NPs in the main text were also applicable to MPs.
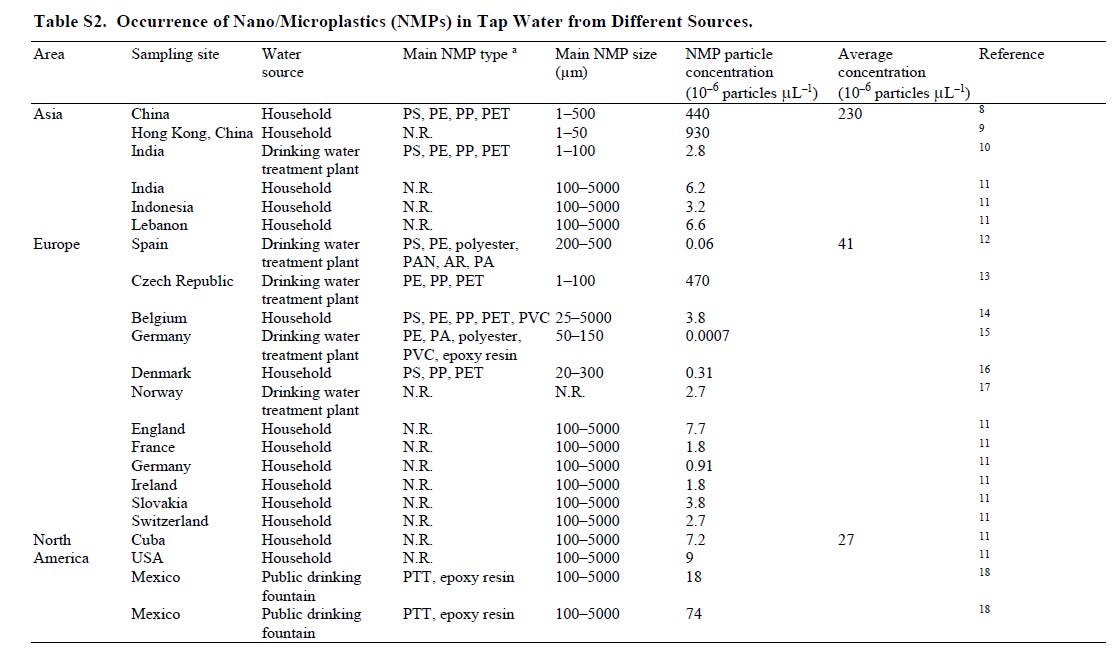
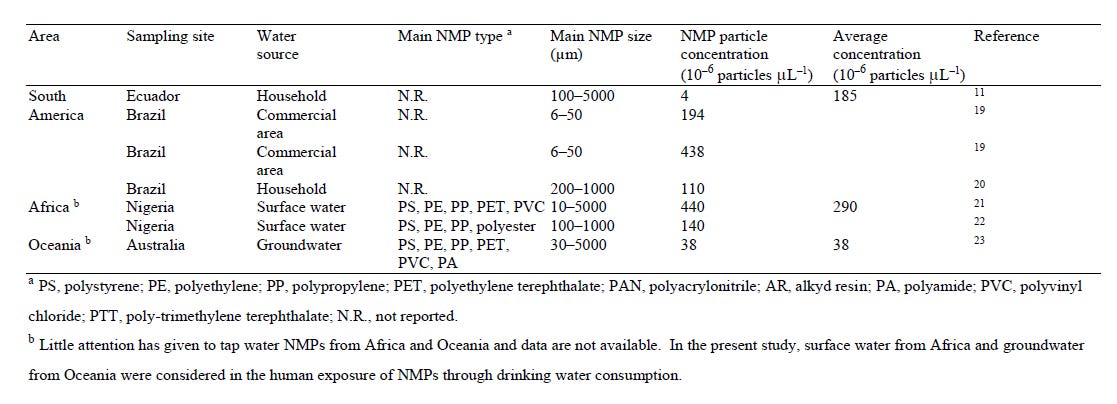
Here are the polymer plastics found in drinking water throughout the world:
Thank you to Karen Kingston, who brought this article to my attention.